Invited Speakers---Dr. Jong-in Hahm

Professor, Department of Chemistry, Georgetown University, USA
Prof. Hahm received her Ph.D. in Chemistry from the University of Chicago and postdoctoral training in Chemistry at Harvard University. She then started a faculty position in the Department of Chemical Engineering at Pennsylvania State University after which she moved to the Department of Chemistry at Georgetown University. She is a recipient of GU Distinguished Achievement in Research Award, KWISE Woman Scientist Award, ACS WCC Rising Star Award, ACS Dreyfus Lectureship Award, and ACS WISE Lectureship Award.
Prof. Hahm’s research interests include nanomaterials, surface and interfacial science, and nanobiotechnology (
http://chemistry.georgetown.edu/people/hahm.html). Her research program is highly interdisciplinary and utilizes the unique properties of polymeric, metallic, and semiconducting nanomaterials (including 1D nanostructures such as nanotubes, nanowires, and nanorods) in advanced biological and biomedical detection. She also conducts fundamental research on biological and polymeric materials at nanoscale. She investigates distinct adsorption and assembly behaviors of individual proteins on nanoscale polymer surfaces to create next-generation biomaterials.
Speech Title: Fundamental Study of Nanoscale Protein-Polymer Interactions and Potential Contributions to Solid-state Protein Nanoarrays
Abstract: This talk presents an overview of our on-going research, aiming to provide fundamental understanding on nanoscale protein adsorption behavior and to develop more advanced, next-generation protein arrays and biomaterial constructs. Intriguing protein adsorption phenomena on nanoscale surfaces exhibiting varying degrees of chemical heterogeneity are directly probed at the individual biomolecule level.
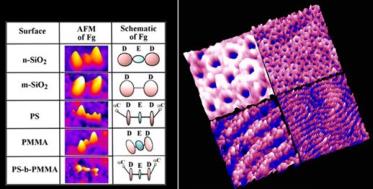
Specifically, we elucidate protein adsorption characteristics on the templates of diblock copolymers, blends, and homopolymers. We also investigate location- dependent protein adsorption behaviors with respect to the size and distance of the interfacial regions defined by different polymer blocks. We carry out bioactivity and biofunctionality measurements of polymeric surface-bound enzymes and compare quantitatively with their free-state activities. We also explore protein assembly on chemically modified, polymeric nanotemplates to provide a range of feature size/shapes in solid-state protein arrays. Our results demonstrate that self-assembling, chemically heterogeneous, nanoscale domains in diblock copolymers can be effectively used for high density protein nanotemplates. Our approach will be particularly beneficial for fabricating periodic patterns of proteins on surfaces with nanometer feature sizes without the use of lithographic techniques based on electron beam or extreme UV. Insight gained from our study may be used to control the surface density, conformation, orientation, and biofunctionality of prebound proteins in highly miniaturized proteomic applications, now approaching nanoscale. For more information, see
https://chemistry.georgetown.edu/Hahm