Biography: Dr. Murat Tas is the Head of the Science Education Department, OndokuzMayis University (OMU), Turkey. He was also affiliated with Karadeniz Technical University and Giresun University in Turkey. Dr. Tas obtained his PhD in Natural Science Institute from OndokuzMayıs University in 2004. Before joining the OndokuzMayıs University, Dr. Tas had worked as a visiting researcher fellow at the University of James cook, Townsville, Australia. The main research areas are synthesis and characterization of nitrogen, phosphorus and sulfur containing organic molecules and their metal complexes. Coordination compounds of some biological active compounds, Heterocyclic ligands, Thermal behaviors of the materials, inorganic polymeric compounds, Porous crystalline materials and MOF’s also works by his groups. The current research interests of his group are in design the controllable porous in the inorganic polymers.
Speech Title: Inorganic Polymers Based on, 3,3ʹ,5,5ʹ-azobenzenetetracarboxylate
Abstract: A large molecule, or macromolecule, composed of many repeated subunits is called as polymer. From familiar synthetic plastics to natural polymers, Polymers are fundamental to biological structure and function. Both synthetic and natural polymers play an essential and ubiquitous role due to their properties and many smaller molecules, called as monomers create the copolymers via polymerization reactions.
Polymer structures containing metal cation centers linked by organic ligands are specifically named as coordination polymer. In fact, a coordination polymer is a coordination compound and the coordination entities repeating to extend in 1, 2, or 3 dimensions.
Such as organic and inorganic chemistry, biochemistry, materials science, electrochemistry, and pharmacology, the coordination polymers have important roles. This interdisciplinary nature has led to extensive work on coordination polymers in the past few decades.
The metal-organic frameworks, or MOFs that are coordination networks with organic ligands having potential voids, are a subclass of coordination polymers. To form MOF’s structures, the one or more metal ion and an organic ligand links the metal ions together into larger arrays. A lot of dicarboxylic acids (i.e., oxalic acid, malonic acid, succinic acid, glutaric acid, terephthalic acid, 1,4-benzenedicarboxylate), tricarboxylic acid (i.e., citric acid, trimesic acid) or azoles (i.e., 1,2,3-triazole, pyrrodiazole) can use to prepare the MOF's.
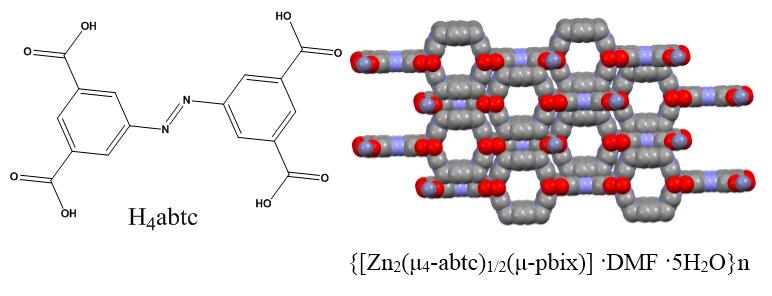
Because of its versatile coordination behaviors the 3,3ʹ,5,5ʹ-azobenzenetetracarboxylic acid (H4abtc) linker has great attention to form MOF's structure in last years. It is in planar nature and has four carboxylic acid groups, so it exhibits promising behavior in the use of secondary ligands for control of voids.